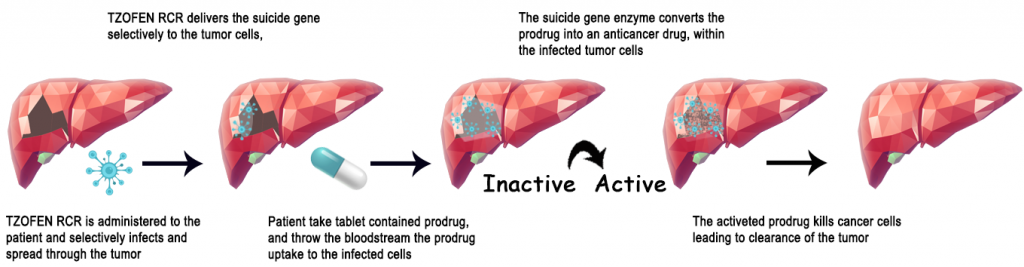
How does it work?
In recent years, oncolytic viruses in general and replication-competent retrovirus (RCR) in particular have emerged as a promising treatment against cancer as monotherapy or in combination with approved checkpoint inhibitors.
At TZOFEN biological therapeutics, we design the future generation of RCR. Our approaches to RCR incorporate several genetic modifications, including:
- Tumor specification
- Two-step cancer cell-killing mechanism
- Ongoing stable infection
TZOFEN RCR is an investigational injectable that encodes a prodrug activator enzyme, and naturally, it does not exist in humans. TZOFEN RCR selectively infects cancer cells so that the infected cancer cells selectively carry the enzyme gene and produce the prodrug activator.

The prodrug is an orally administered FDA approved drug. Regarding cancer, it acts as an inactive prodrug. When follow-up isorally administered, the prodrug is absorbed and carried through the bloodstream and diffuses into the cancer cells. In animal models, TZOFEN has proven that the prodrug is converted at high concentrations into the active anticancer drug within the infected cancer cells.